FDA approved home use walking technologies:

PheoniX by SuitX
Pheonix by SuitX exoskeleton is the world's lightest and most advanced exoskeleton designed to help people with mobility disorders to be upright and mobile.

Indego Exoskeleton
Indego exoskeleton orthotically fits to the lower limbs and the trunk; the device is intended to enable individuals with spinal cord injury at levels T3 to L5 to perform ambulatory functions with supervision of a specially trained companion in accordance with the user assessment and training certification program.
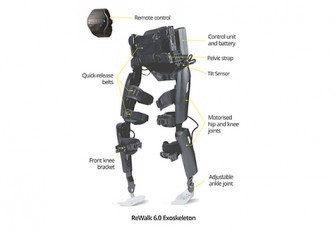
ReWalk Personal
ReWalk Personal exoskeleton battery-powered system features a light, wearable exoskeleton with motors at the hip and knee joints. The ReWalker controls movement using subtle changes in his/her center of gravity. A forward tilt of the upper body is sensed by the system, which initiates the first step. Repeated body shifting generates a sequence of steps which mimics a functional natural gait of the legs. It is the first FDA approved home use exoskeleton.

Bioness L300 FES
Bioness L300 functional electrical stimulation (FES) foot drop and thigh weakness devices. A 3-axis gyroscope and accelerometer are embedded in the Stimulator to monitor user movement in all three kinematic planes and deploy stimulation in 0.01 seconds of detecting a valid gait event.
Through an adaptive, learning algorithm, the L300 Go detects gait events, providing stimulation precisely when needed making it easier for users to clear their foot at different walking speeds, on stairs, ramps, and while navigating uneven terrain.

Ottobock E-MAG Active
Ottobock E-MAG Active electromagnetic stance control orthosis acts as an active walking partner, not just a static support. E-MAG Active is calibrated to you, and throughout your walking motion – or phase of gait. The sensor integrated into the electronics uses data to unlock the knee joint at the right point of your gait.

Ottobock C brace
Ottobock C brace mechatronic stance and swing phase control orthosis system supports you through the entire gait cycle and adapts to everyday situations in real-time. The microprocessor sensor makes the entire gait pattern more dynamic and responsive. The user can also change settings on their joint: switching to cycling mode, using the smartphone app.
Emerging Technologies not yet FDA approved for home use:

Cyberdyne's HAL
Cyberdyne’s Hybrid Assisted Limb or HAL, available to U.S. patients. The exoskeleton, which is currently available through licensed medical facilities only, uses sensors to detect bioelectric signals sent from your brain to your muscles, which it pairs with your movement (or intended movement) in order to increase strength and stability.

EksoNR
EksoNR is a robotic exoskeleton specifically designed to be used in a rehabilitation setting to progress neurorehab patients so they can walk out of the device and back into their communities. As the first exoskeleton FDA-cleared for acquired brain injury, stroke, and spinal cord injury, EksoNR offers the industry’s most natural gait, re-teaching the brain and muscles how to properly walk again.
And more walking technologies continue to be developed.



